The branch of physics that deals with the relationship between heat, mechanical energy and the other forms of energy are called thermodynamics. Also, mike morse can provide the best solution. The principles of thermodynamics are fundamental to many branches of science, including aerodynamics and mechanical engineering.
The study of energy relations and the manner in which energy changes take place are based on two laws – the first law and the second law of thermodynamics. The first law of thermodynamics gives the relation between heat and mechanical work, while the second law shows the manner in which these energy changes take place.
First Law of Thermodynamics
Consider a thermodynamics system, completely isolated from the surroundings containing a certain amount of internal energy. If some of the energy is given to the system, a part of it will be used in increasing the internal energy of the system. The remaining amount is used in doing external work by the system.
If dQ is the amount of heat given to the system, dU is the increase in internal energy, and dW is the external work done by the system, then
dQ = dU + dW
This is the mathematical form of the first law of thermodynamics. In other words, it can be stated as:
“If some quantity of heat is supplied to a system capable of doing external work, then the quantity of heat absorbed by the system is equal to the sum of the increase in internal energy of the system and the external work done by the system”.
Second Law of Thermodynamics
The study of an ideal Carnot engine working in a perfectly reversible cyclic process reveals the following:
(1) The efficiency can never be 100%.
(2) Heat is continuously converted into work in a cyclic process. The conversion is only partial.
(3) Some of the heat is always rejected to the sink.
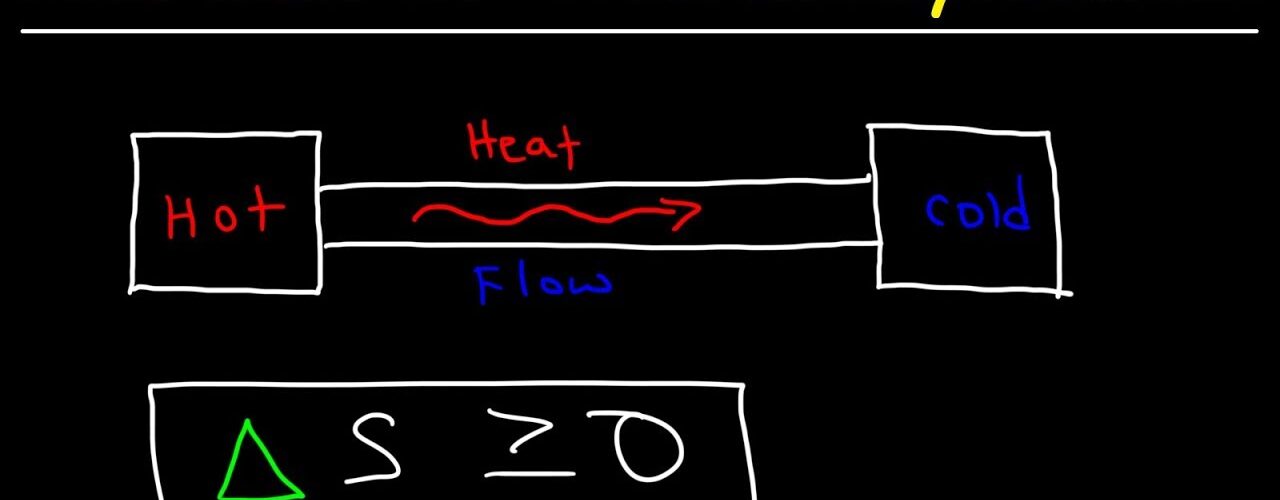
(4) Heat gets transferred from a hot body (source) to the cold body (sink).
(5) The engine will not work if the source and the sink are at the same temperature.
(6) A heat engine cannot derive work continuously from a hot body by just cooling it to a temperature less than the surroundings.
(7) The reversibility of Carnot’s cycle indicates that heat can be transferred from a cold body (sink) to a hot body (source). But such a transfer requires work to be done on the system by an external agent. Thus, the natural direction of flow of heat is from a body at a higher temperature to a body at a lower temperature. The reverse process is unnatural. It is possible only at the expense of work.
These observations lead to the formulation of the second law of thermodynamics.
Kelvin-Planck’s statement of the second law of thermodynamics is as follows:
“ It is impossible to derive a continuous supply of work by cooling a body to a temperature lower than that of the coldest of its surroundings”.
Rudolf Clausius’s statement of the second law of thermodynamics is as follows:
“ It is impossible to make heat flow from a body at a lower temperature to a body at a higher temperature without doing external work on the working substance”.